New DED treatment regime on the horizon?
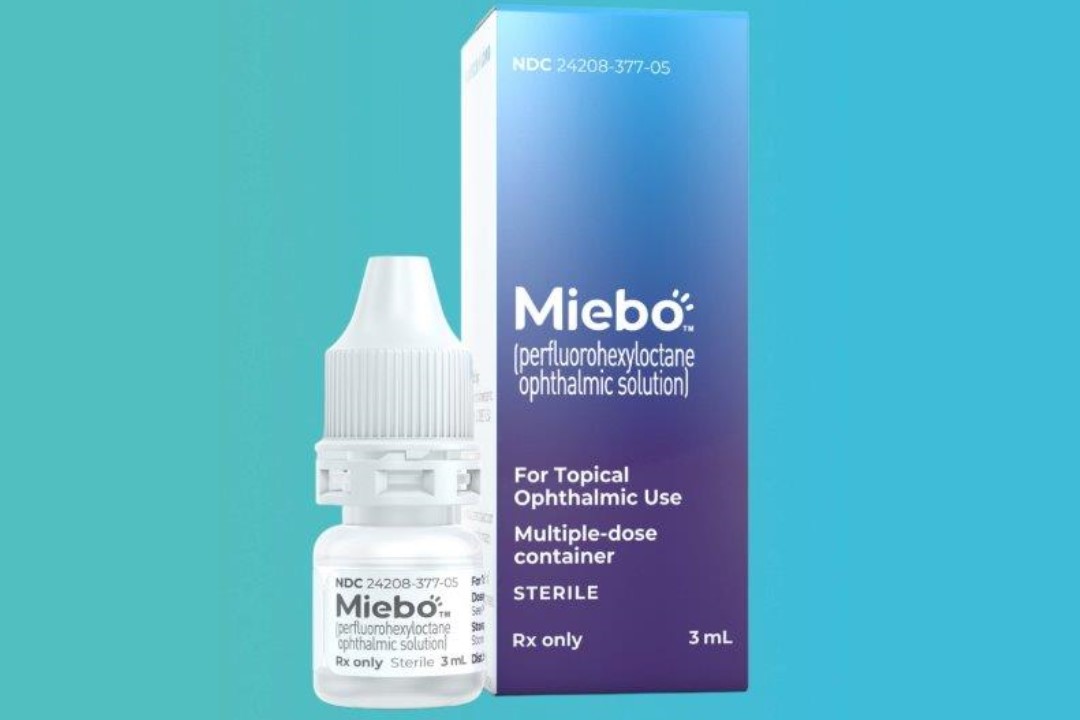
Miebo, a single-ingredient tear stabiliser from Bausch + Lomb and Novaliq, has been given the green light by the US Food and Drug Administration (FDA), offering a new treatment for dry eye disease (DED).
Formerly known as NOV03, Miebo is the first FDA-approved therapeutic directly targeting the excessive tear evaporation often associated with meibomian gland dysfunction (MGD). Its only component is perfluorohexyloctane (F6H8), a semifluorinated alkane which stabilises the tear film on the surface of the eye, reducing tear evaporation and drying of the eyes.
The approval was supported by positive data from the GOBI and MOJAVE phase 3 pivotal clinical trials, which included 1,217 patients with DED and clinical signs of MGD. Miebo was reported to have consistently met primary clinical signs (change in eye dryness and corneal fluorescein staining) and patient-reported symptom endpoints in the trials.
“Tear evaporation, a leading driver of DED, presents a significant treatment challenge. With the approval of Miebo, eyecare professionals can now take a new approach to DED therapy with a first-in-class water- and preservative-free prescription treatment that specifically addresses tear evaporation,” said Dr Paul Karpecki, Cornea and External Disease director at the Kentucky Eye Institute, and associate professor at the University of Pikeville, Kentucky College of Optometry.
Bausch + Lomb and Novaliq said they expect to make Miebo commercially available in the US the second half of this year.
In Australia and New Zealand, perfluorohexyloctane (F6H8) is marketed by AFT Pharmaceuticals as Novatears.