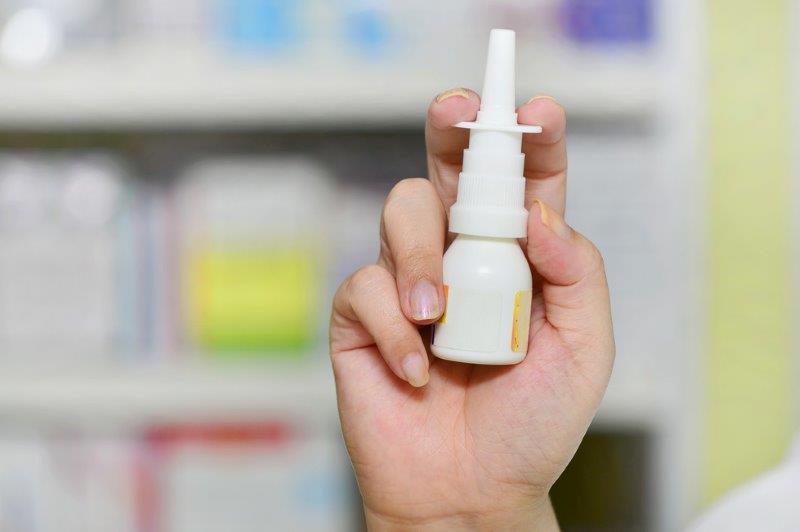
Nasal spray for dry eye near
February 12, 2021
Staff reporters
Oyster Point Pharma has submitted an application to the US Food and Drug Administration (FDA) for its OC-01 (varenicline) nasal spray for the treatment of signs and symptoms of dry eye disease.
The new drug application is supported by safety and efficacy results from multiple clinical trials conducted in 1000+ patients with a broad range of dry eye disease.
“We are excited by the potential of OC-01 nasal spray, if approved, becoming a meaningful addition to the eye care practitioner’s treatment armamentarium, and the potential to create a paradigm shift in how practitioners and their patients treat dry eye disease,” said Dr Marian Macsai, Oyster Point Pharma’s chief medical officer.