Approaches to revitalising or replacing the corneal endothelium
The corneal endothelium is a monolayer lining the back of the cornea and is responsible for maintaining corneal transparency. Human corneal endothelial cells (CECs) have limited proliferative and regenerative capacities because they are arrested in the G1 phase of the cell cycle. Due to the low regenerative capacity and degeneration over time, loss of CECs in the adult human eye is compensated by size expansion of the remaining cells. For healthy adults aged 20–39 years, the average CEC density is around 3,000 cells/mm2, which declines by 0.3% annually1. To preserve corneal transparency, endothelial cell density must remain above a critical threshold, usually 400-500 cells/mm2. Loss or dysfunction of CECs, such as in Fuchs’ endothelial corneal dystrophy (FECD) and pseudophakic bullous keratopathy (PBK), leads to endothelial decompensation, corneal oedema and eventually loss of vision. FECD has a global estimated prevalence of 4-9% in those aged over 50 years old and PBK occurs in 1-2% of patients receiving cataract surgeries. In New Zealand, corneal endothelial diseases, especially FECD, have become more prevalent in recent years2. Therefore, approaches to revitalising or replacing the corneal endothelium are important to improving eye health outcomes. Currently, most of the approaches for corneal endothelial regeneration which have been studied or are under development include surgical treatment, cell therapies, acellular graft substitutes, pharmacological and genetic modulation (Fig 1).

Fig 1. Approaches for corneal endothelium regeneration. Credit: Pere Català et al (2022) CC BY1
Surgical treatment
For end-stage corneal endothelial diseases, the widely utilised treatment is corneal transplantation, which allows replacement of corneal lesions with human donor corneal tissues or other alternative sources. Penetrating keratoplasty (PK) used to be the prevalent treatment modality, but it is restricted by intraoperative complications related to the open-sky procedure and postoperative graft rejections. Endothelial keratoplasty (EK), including Descemet stripping (Automated) endothelial keratoplasty (DS(A)EK) and Descemet membrane endothelial keratoplasty (DMEK), enables replacement of the diseased endothelium with a donor corneal endothelium and Descemet membrane with/without some corneal stroma, in a closed-eye procedure. Compared with PK, EK offers faster recovery of vision and lower incidence of intraoperative complications and postoperative graft rejection3. EK has now become the primary therapy in the treatment of various corneal endothelial diseases. Data from the New Zealand National Eye Bank over the past two decades have reported a significant increase of EK in total corneal transplantations (Fig 2)2. Although keratoplasty can effectively restore corneal transparency, it is, however, limited by the severe donor shortage in some regions and countries. Therefore, alternatives are required.

Fig 2. Number of corneal transplantations, New Zealand National Eye Bank 1991–2015.
Credit: Bia Kim et al (2017)2
Recently, a novel technique known as Descemet stripping only (DSO) or Descemetorhexis without endothelial keratoplasty (DWEK) has shown promising results in some clinical trials. It involves removal of the central corneal endothelium without subsequent transplantation. This technique only works for selected cases with localised central oedema (around 4mm) and relatively young patients with healthy peripheral endothelium4. Further development, such as the use of pharmacological modulation or acellular corneal endothelial graft equivalents, may be required to improve the success rate in patients with larger lesions and lower peripheral CEC density.
Endothelial cell therapy
Endothelial cell therapy aims to implant cultivated CECs, through intraocular injection or on a tissue-engineered substrate, to replace the damaged endothelium. Transplantation of cultured CECs is a feasible strategy to alleviate donor cornea shortage. For a long time, the ex vivo culture of CECs has been hindered by contact inhibition and endothelial-mesenchymal transition, where cells take on a fibroblastic phenotype and become highly proliferative, but lose endothelial function. With advances in understanding of CEC biology, it is now possible to cultivate CECs in vitro, thus providing new opportunities for the development of endothelial cell therapy. The proliferative capacity of CECs could be stimulated by Rho-associated kinase (ROCK) inhibitors and nuclear catenin p120 in vitro. The injection of human CECs supplemented with Y-27632, a ROCK inhibitor, in the anterior chamber, has achieved good clinical efficacy in the treatment of bullous keratopathy, including recovery of endothelial cell density, corneal transparency and visual acuity5. Moreover, blockage of the TGF-β pathway by SB431542 (a selective inhibitor of TGF-β receptor) has helped reduce the fibroblastic phenotype of cultivated CECs. At the moment, CEC primary culture is still limited by the requirement for young donor corneas, which are relatively rare.
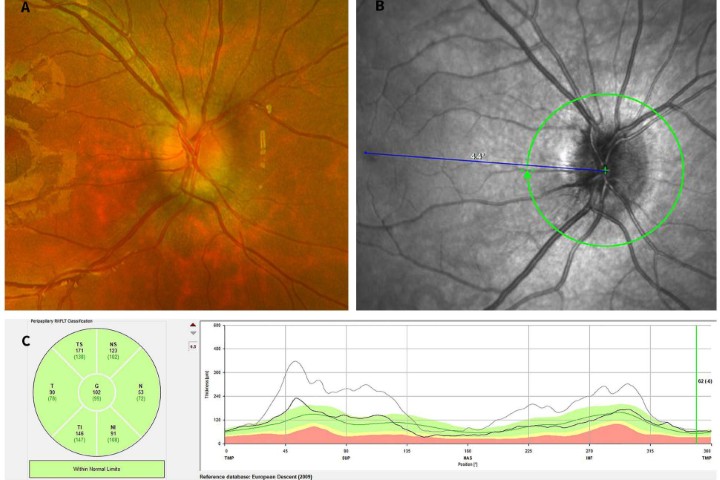
Recent evidence has suggested the existence of corneal endothelial stem cells. It is believed endothelial stem cells reside in the transition zone (TZ), which encompasses the area between the edge of the peripheral endothelium and the beginning of Schlemm’s canal, with Schwalbe’s line in the middle6. Our recent study successfully established the primary culture of TZ cells from redundant human limbal tissues after corneal transplantation. Human TZ cells can proliferate and differentiate into CEC-like cells and express functional CEC markers (Fig 3)7. In the future, intracameral injection or transplantation using tissue-engineering sheets of corneal endothelial stem cells might promote endothelial function for intractable corneal endothelial diseases.

Fig 3. Primary culture of transition zone (TZ) cells and expression of stem cell (Nestin) and corneal endothelial markers (ZO-1, Na+/K+ ATPase) in TZ cells. Credit: Jie Zhang et al (2020)7
Compared to primary cultured CECs, alternative cellular sources can shorten cell expansion time and potentially tackle the global donor tissue scarcity. Recently, pluripotent stem cells, namely embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have shown promising results in animal studies. The injection of ESC/iPSC-derived CECs into the anterior chamber demonstrated efficacy in reforming a functional corneal endothelium on denuded Descemet membrane in animal models. However, iPSCs face significant challenges, such as difficulty of differentiation control and the possibility of tumorigenesis, and ethical issues strictly prohibit ESC use in many countries8. Other CEC sources, such as bone-marrow-derived endothelial precursors, neural crest cells, skin-derived precursors and mesenchymal stem cells, have also shown feasible results in both cell and animal studies. Despite the encouraging outcomes, further studies are still needed to test their functional stability, long-term safety, and interactions with native corneal endothelium.
Acellular corneal endothelial graft substitutes
Acellular corneal endothelial graft substitutes aim to promote the proliferation and migration of peripheral CECs toward the central acellular regions after central corneal endothelial stripping, such as in DSO/DWEK surgeries. Acellular corneal endothelial graft substitutes have shown favourable outcomes, including improved visual acuity, reduced corneal thickness and increased CEC density in a patient with FECD who received acellular Descemet membrane transplantation after the central 5.0mm of diseased endothelium was stripped9. Recently, a synthetic endothelial graft substitute (EndoArt) was reported to be effective in improving central corneal transparency and visual acuity in five patients with failed repeat EK surgeries10. The outstanding advantages of acellular corneal endothelial graft substitutes are that they are independent from donor corneas and less likely to develop corneal infections and immune rejections postoperatively. But larger scale clinical studies are needed to confirm long-term implant adhesion to host cornea.
Pharmacological modulation of the corneal endothelium
Pharmacological modulation of the corneal endothelium has demonstrated promising results in promoting CEC survival, proliferation and migration in the treatment of various corneal endothelial diseases after DSO/DWEK surgeries. ROCK inhibitors, including Y-27632 and ripasudil, have shown success in clinical trials. Several studies have reported the therapeutic effectiveness of Y-27632 in treating FECD and bullous keratopathy11,12. The repopulation of CECs following the use of ripasudil eye drops in two patients with FECD whose corneas did not clear two months after a DSO/DWEK procedure confirmed its efficacy in improving CEC proliferation and migration13. Moreover, an engineered human fibroblast growth factor 1 protein, TTHX1114, has also shown potential to trigger corneal endothelial regeneration in organ-cultured human corneas after DSO/DWEK procedures. Despite successful preliminary results, the efficacy of pharmacological modulation of the corneal endothelium still needs larger randomised control trials to compare with a DSO/DWEK-only group. The identification of appropriate patients and optimal drug dosage and therapeutic duration also remain to be explored.
Genetic modulation of the corneal endothelium
Genetic alterations are one of the leading causes of corneal endothelial dystrophies, ie, posterior polymorphous corneal dystrophy (PPCD), congenital hereditary endothelial dystrophy (CHED), and FECD. PPCD is an autosomal dominant disorder and reported to be associated with mutations in four genes: OVOL2, COL8A2, ZEB1 and GRHL2. CHED is an autosomal recessive disorder, primarily related to SLC4A11 mutation. FECD is found to be linked with multiple genetic variations, ie, COL8A2, TCF4, SLC4A11, and ZEB114. The application of genome sequencing can help identify the pathogenic genetic changes in patients with inherited corneal endothelial dystrophies. Developing tools for correcting these genomic alterations or avoiding their associated effects, such as using adeno-associated viral vectors, siRNA, miRNA and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system, could potentially rectify the genetic flaws behind the disease and avoid corneal transplantation, sparing donor corneas for other refractory corneal diseases1. But since genetic modulation of the corneal endothelium is still in an early stage of development, further studies are required to assure the long-term efficacy and safety of this therapy.
Conclusion
Corneal endothelial diseases have become one of the leading indications for corneal transplantations. EK surgeries, namely DS(A)EK and DMEK, can effectively restore corneal transparency, but rely on the availability of donor cornea tissues. Endothelial cell therapy using cultured CECs or other sources is a feasible strategy to overcome donor cornea shortage. DSO/DWEK has demonstrated encouraging outcomes in selected patients with localised central oedema, without the need of donor tissue or cells, but the wider application may require the use of pharmacological modulation or acellular corneal endothelial graft substitutes. Genetic modulation could correct the genotype behind the disease and save the donor tissues for those in need. Overall, these therapies hold the tantalising hope that diseased corneal endothelium can be revitalised or replaced without complications.
References
1. Catala P, Thuret G, Skottman H, et al. Approaches for corneal endothelium regenerative medicine. Prog Retin Eye Res 2022; 87: 100987.
2. Kim BZ, Meyer JJ, Brookes NH, et al. New Zealand trends in corneal transplantation over the 25 years 1991-2015. Br J Ophthalmol 2017; 101(6): 834-8.
3. Ong HS, Ang M, Mehta J. Evolution of therapies for the corneal endothelium: past, present and future approaches. Br J Ophthalmol 2021; 105(4): 454-67.
4. Borkar DS, Veldman P, Colby KA. Treatment of Fuchs Endothelial Dystrophy by Descemet Stripping Without Endothelial Keratoplasty. Cornea 2016; 35(10): 1267-73.
5. Kinoshita S, Koizumi N, Ueno M, et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N Engl J Med 2018; 378(11): 995-1003.
6. Xiao Y, McGhee CNJ, Zhang J. Adult stem cells in the eye: Identification, characterisation, and therapeutic application in ocular regeneration - A review. Clin Exp Ophthalmol 2024; 52(2): 148-66.
7. Zhang J, Ahmad AM, Ng H, Shi J, McGhee CNJ, Patel DV. Successful culture of human transition zone cells. Clin Exp Ophthalmol 2020; 48(5): 689-700.
8. Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. Stem c ells: past, present, and future. Stem Cell Res Ther 2019; 10(1): 68.
9. Soh YQ, Mehta JS. Regenerative Therapy for Fuchs Endothelial Corneal Dystrophy. Cornea 2018; 37(4): 523-7.
10. Fontana L, di Geronimo N, Cennamo M, Mencucci R, Versura P, Moramarco A. Early Outcomes of an Artificial Endothelial Replacement Membrane Implantation After Failed Repeat Endothelial Keratoplasty. Cornea 2023.
11. Koizumi N, Okumura N, Ueno M, Nakagawa H, Hamuro J, Kinoshita S. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea 2013; 32(8): 1167-70.
12. Okumura N, Inoue R, Okazaki Y, et al. Effect of the Rho Kinase Inhibitor Y-27632 on Corneal Endothelial Wound Healing. Invest Ophthalmol Vis Sci 2015; 56(10): 6067-74.
13. Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis Without Grafting for Fuchs Endothelial Dystrophy-Supplementation With Topical Ripasudil. Cornea 2017; 36(6): 642-8.
14. Zhang J, McGhee CNJ, Patel DV. The Molecular Basis of Fuchs' Endothelial Corneal Dystrophy. Mol Diagn Ther 2019; 23(1): 97-112.

Dr Yuting Xiao is an ophthalmologist in China and a PhD student at the University of Auckland, supervised by Professor Charles McGhee and Dr Jie Zhang. Her research involves exploring the potential regenerative effect of limbal mesenchymal stem cells on corneal endothelial stem cells from the transition zone.

Dr Jie Zhang is a Senior HRC Research Fellow at the Department of Ophthalmology, University of Auckland. She has specific research interests in both laboratory and clinical aspects of the cornea and anterior segment.